Khalimonchuk Lab
Welcome to the Khalimonchuk Lab at the University of Nebraska
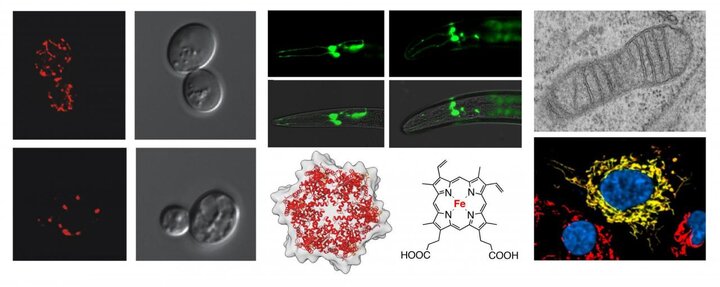
Work in our lab is focused on fundamental biological processes that involve mitochondria. To gain insights into vital aspects of mitochondrial biology and human disease and aging, we utilize several model systems and state-of-the-art genetics and biochemical approaches as well as various physiological and imaging techniques.
Department of Biochemistry
University of Nebraska
N230 Beadle Center
Lincoln, NE 68588-0664
402-472-8060
Research Overview:
Mitochondria are complex and highly dynamic organelles responsible for a number of vital functions including cellular energy conversion, a plethora of metabolic and biosynthetic pathways, maintenance of ion homeostasis and cell death. Perturbations to mitochondrial function and integrity lead to dysfunctions that manifest in a spectrum of early- to adult-onset neurological and cardiovascular disorders, certain types of cancer, Type II diabetes and neurodegeneration. Understanding the molecular bases of mitochondrial function/dysfunction is a key to finding ways to combat these currently incurable diseases. Our research utilizes yeast, roundworm and mammalian cell culture models to address the following fundamental questions.
Role of protein quality control in mitochondrial homeostasis and stress responses
Mitochondrial respiration is inherently linked to generation of reactive oxygen species (ROS). In addition, redox-active intermediates in the biogenesis of the electron transfer chain respiratory complexes can further facilitate ROS production. Accumulating or persisting ROS can damage mitochondrial proteins and/or DNA located in the vicinity to respiratory chain, thereby contributing to mitochondrial dysfunction. Mitochondrial protein quality control (MPQC) system composed of molecular chaperones and proteases is a key factor that helps cells to cope with homeostatic challenges such as oxidative damage and protein misfolding. MPQC comprises a number of highly conserved proteases and molecular chaperones, important functions of which remain obscure. We seek to elucidate the individual roles of various MPQC components in preservation of mitochondrial functions and plasticity, and determine how impaired protein processing or turnover leads to aging-related diseases.
Mitochondrial heme transport
Heme is an essential, but inherently reactive and cytotoxic cofactor and signaling molecule. In most eukaryotes heme biosynthesis is initiated and completed within the mitochondria. All mitochondrial heme species are generated from heme b (aka protoheme) produced by the enzyme ferrochelatase and must be mobilized and trafficked for further distribution in virtually every subcellular compartment via largely unknown mechanisms. Our studies are aimed at understanding the mechanisms that govern safe mobilization, modification, transport and distribution of heme within and out of the mitochondria. Unraveling the molecular details of heme trafficking and export in health and disease states may identify targetable molecules for treatment of highly prevalent human disorders associated with heme and iron dyshomeostasis.
Biogenesis and maintenance of protein complexes within the inner mitochondrial membrane
The vast majority of proteins comprising mitochondrial proteome is synthesized in the cytosol and imported into the organelle, while only a handful of polypeptides originate from the mitochondrial genome. The inner mitochondrial membrane (IM) is an ultimate destination for many of these proteins wherein they are organized into high molecular weight complexes. Some of these assemblies such as cytochrome c oxidase (aka Complex IV), encompass proteins of dual genomic origin and harbor highly reactive prosthetic groups. To ensure normal mitochondrial function, numerous dedicated chaperones and assembly factors assist and regulate biogenesis and maintenance of these protein machineries. We seek to understand how the IM-localized multiprotein ensembles are formed and maintained, and how their erroneous biogenesis due to mutations in assembly factors drives clinical manifestations.
Publications
PubMed Link
Complete list of our published work can be found at:
https://scholar.google.com/citations?user=k2OzIN0AAAAJ&hl=en&oi=ao
http://www.ncbi.nlm.nih.gov/myncbi/oleh.khalimonchuk.1/bibliography/public/
Selected Recent Publications:
1. Nyvltova, E., Dietz, J.V., Seravalli, J., Khalimonchuk, O., Barrientos, A. (2022) Coordination of metal center biogenesis in human cytochrome c oxidase. Nature Commun. 13, 3615.
2. Dietz, J.V., Bohovych, I., Willoughby, M.M., Piel 3rd, R.B., Ross, T.A., Addis, H.G., Fox, J.L., Lanzilotta, W.N., Dailey, H.A., Wohlschlegel, J.A., Reddi, A.R., Medlock, A.E., Khalimonchuk, O. (2021) Mitochondrial contact site and cristae organizing system (MICOS) machinery supports heme biosynthesis by enabling optimal performance of ferrochelatase. Redox Biol. 46, 102125.
3. Viana, M.P., Levytskyy, R.M., Anand, R., Reichert, A.S., Khalimonchuk, O. (2021) Protease OMA1 modulates mitochondrial bioenergetics and ultrastructure through dynamic association with MICOS complex iScience 24, 102119.
4. Martinez-Guzman, O., Willoughby M.M., Saini, A., Dietz, J.V., Bohovych, I., Medlock, A.E., Khalimonchuk, O., Reddi, A.R. (2020) Mitochondria-nuclear heme trafficking is regulated by GTPases that control mitochondrial dynamics and ER contact sites. J. Cell Sci. jcs.237917.
5. Daverey, A., Levytskyy, R.M., Swenson, S., Hayward, S.L., Stanke, K.M., Viana, M.P., Narasimhan, M., Khalimonchuk, O., Kidambi, S. (2019) Depletion of mitochondrial protease OMA1 alters proliferative properties and promotes metastatic growth of breast cancer cells. Sci. Rep. 9, 14746.
6. Bohovych, I., Dietz, J.V., Swenson, S., Zahayko, N., Khalimonchuk, O. (2019) Redox regulation of the mitochondrial quality control protease Oma1. Antioxid. Redox Signal. 31, 429-443.
7. Germany, E.M., Zahayko, N., Huebsch, M., Fox, J.L., Prahlad, V., Khalimonchuk, O. (2018) The AAA-ATPase Afg1 preserves organellar fidelity and cellular healthspan by maintaining mitochondrial matrix proteostasis. J. Cell Sci. 131, pii: jcs219956.
8. Tsushima, K., Bugger, H., Wende, A.R., Soto, J., Jenson, G.A., Tor, A.R., McGlauflin, R., Kenny, H.C., Zhang, Y., Souvenir, R., Hu, X.X., Sloan, C.L., Pereira, R.O., Lira, V.A., Spitzer, K.W., Sharp, T.L., Shoghi, K.I., Sparagna, G.C., Rog-Zielinska, E.A., Kohl, P., Khalimonchuk, O., Schaffer, J.E., Abel, E.D. (2018) Mitochondrial reactive oxygen species in lipotoxic hearts induce post-translational modifications of AKAP121, DRP1 and OPA1 that promote mitochondrial fission. Circ. Res. 122, 58-73.
9. Taylor, N.G., Swenson, S., Harris, N.J., Germany, E.M., Fox, J.L., Khalimonchuk, O. (2017) The assembly factor Pet117 couples heme a synthase activity to cytochrome oxidase assembly. J. Biol. Chem. 292, 1815-1825.
10. Bohovych, I., Kastora, S., Christianson, S., Kim, H.J., Fangman, T., Zhou, Y.J., Barrientos, A., Brown, A.J., Khalimonchuk, O. (2016) Oma1 links mitochondrial protein quality control and TOR signaling to modulate physiological plasticity and cellular stress responses. Mol. Cell. Biol. 36, 2300-2312.
Past Postdoctoral Fellows
Ganapathi Kandasamy, PhD, University of Cologne
Present position: Postdoctroal Researcher, University of Miami
Sang-Gyu Hwang, PhD, University of Ulsan College of Medicine
Present position: Research Scientist, University of Ulsan, South Korea
Roman Levytskyy, PhD, MBA, UCSD
Present position: Director of Scientific Communications & Publications, Nurix Therapeutics Inc.
Kacoli Sen Banerjee, PhD, Indian Institute of Technology
Present position: Research Associate, University of Florida
Past Graduate Students
Timothy Hackett, PhD '23
(co-advised with Dr. Sri Kidambi, Chemical & Biomolecular Engineering)
Present position: Staff Scientist, Charles River Laboratories
Kimberly Stanke, PhD '21
(co-advised with Dr. Sri Kidambi, Chemical & Biomolecular Engineering)
Present position: Instructor in Statistics, University of Nebraska-Lincoln
Jonathan Dietz, PhD '21
Present position: Postdoctoral Fellow, University of Chicago
Martonio Ponte Viana, MS '20
Present position: Senior Associate Scientist, Pfizer Inc.
Edward Germany, PhD '19
Present position: Research Scientist, Nectagen Inc.
Samantha Swenson, PhD '17
Present position: Instructor, University of Nebraska Medical Center
Christina Davis Wilson, PhD '16
(co-advised with Dr. Sri Kidambi, Chemical & Biomolecular Engineering)
Present position: Research Associate, Doane University
Past Undergraduate Students
Zaina Nasser, BS '25
Applying to medical schools
Gabriella "Gabi" Menezes da Silva, BS '24
Applying to medical schools
Zoe Keese, BS '23
MD student, University of Nebraska Medical Center
Lilly Zhou, BS '23
MPH student, University of Nebraska Medical Center
Elinor Stanley, BS '22
DDS student, Creighton University School of Dentistry
Andrew "Drew" Harrahill, BS '21
MD/PhD student, University of Utah School of Medicine
Jooeun "Jenny" Song, BS '21
MD, Psychiatry Resident Physician, Charles R. Drew University / LA County Department of Mental Health
Colton Roessner, BS '17
MD, Anesthesiologist, Ohio State University Hospital
Danelle Topil, BA '17
Catholic Teacher Corps Program at Creighton University
Sara Christianson, BS '15
DO, Family Medicine Physician, Avera McKennan Hospital & University Health Center
Garrett Donaldson, BS '15
MD, Family Medicine Resident, Pella Regional Health Center
Andrew Cannon, BS '13 (NWU)
MD/PhD, Pathology Resident, Mayo Clinic
Past Visiting Researchers & Interns
Marwa Al-Lami, Research Intern, UNL
Vincent Barger, Research Intern, UNL
Mackenzie Allen, Summer Research Intern, Creighton University
Han Le, INBRE Research Intern, Nebraska Wesleyan University
Mustafa Al-Barazanji, Post-baccalaureate Research Intern, UNL
Solena Hessel, REU student, California State University, Long Beach
Kristine Hoagstrom, INBRE Summer Research Intern, Nebraska Wesleyan University
Guy Kunzmann, REU Student, North Carolina State University
Tasloach Wol, REU Student, University of Nebraska-Omaha
Christopher Kastner, REU Student, Nebraska Indian Community College







